by Frank Bergman, Slay News:

The pilot of a packed passenger jet became “incapacitated” during a flight from the UK to Turkey, causing the plane to make an emergency landing in Hungary.
The Jet2 Boeing 737-800, registration G-GDFP, was performing flight LS-1711 from Manchester, England to Dalaman, Turkey with 161 passengers and 6 crew on board.
The plane was enroute at FL370 about 80nm south-southwest of Budapest, Hungary when the crew put out an emergency call.



 Support weight loss with a solution derived from Mother Earth:
Support weight loss with a solution derived from Mother Earth: 


 – Healing From The Globalists Bioweapons
– Healing From The Globalists Bioweapons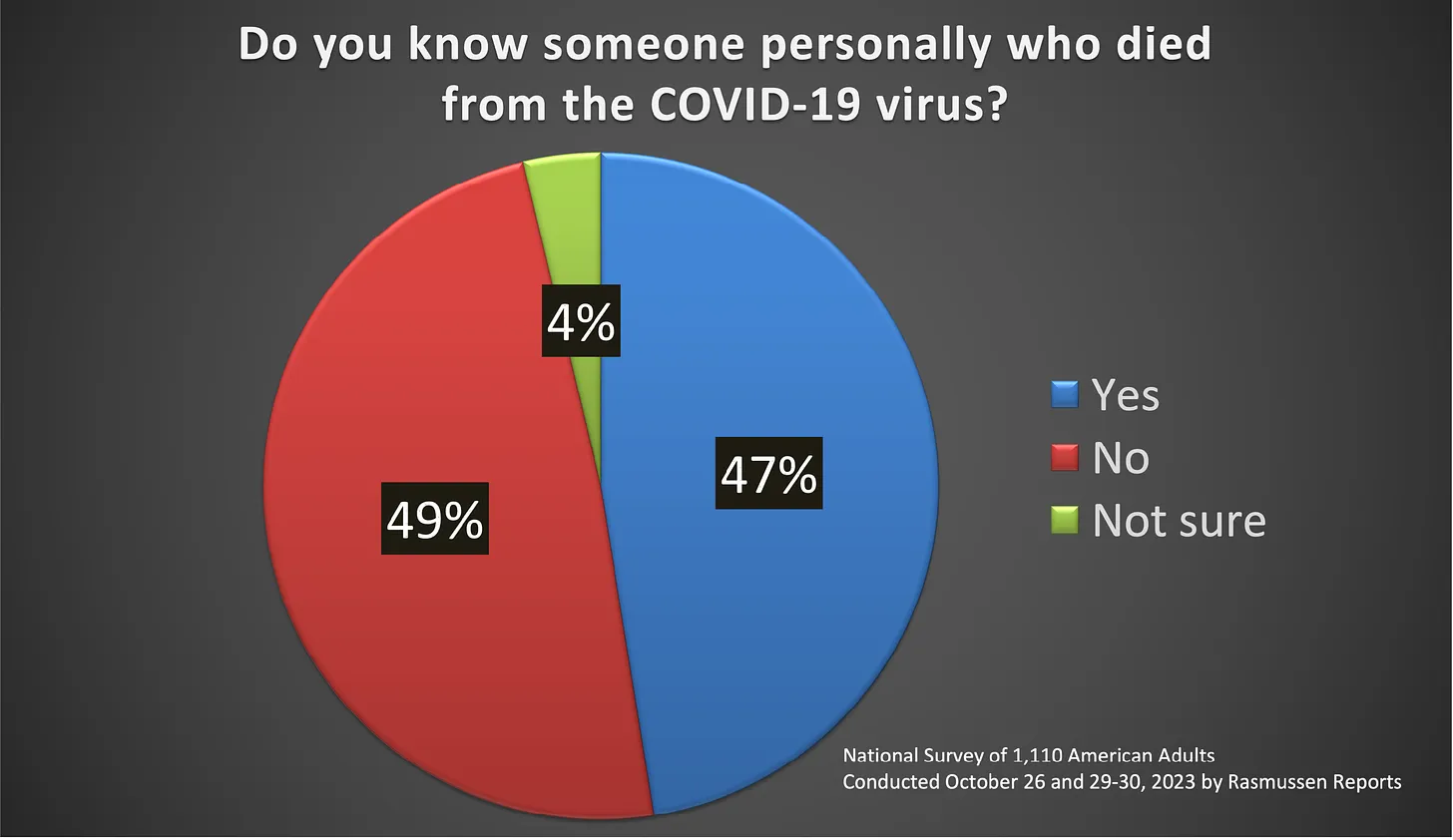 Wow. 42% of Americans were vaccine injured! The poll also implies that 500,000 Americans were killed by the jab. How many more Americans must be killed before this deadly vaccine is stopped?
Wow. 42% of Americans were vaccine injured! The poll also implies that 500,000 Americans were killed by the jab. How many more Americans must be killed before this deadly vaccine is stopped?



 The French study included 30,202 patients.
The French study included 30,202 patients.