by Will Jones, Daily Sceptic:

Synthetic plasmid DNA contamination has been detected in Australian vials of Pfizer and Moderna Covid vaccines at levels of between seven and 145 times the allowable limit, a new study shows.
The independent study of three modified RNA (mod-RNA) vaccine vials, including lots for children and adults, was commissioned to provide evidence in a Federal Court lawsuit over the validity of the regulatory status of the vaccines.
The case, brought by legal firm P.J. O’Brien & Associates, alleges that the vaccines contain unlicensed genetically modified organisms (GMOs) in the form of synthetic DNA contamination and mod-RNA-LNP complexes which could pose an untested safety risk, including the potential for DNA integration into the human genome.
TRUTH LIVES on at https://sgtreport.tv/
In an affidavit provided to legal firm P.J. O’Brien & Associates, molecular virologist Dr. David Speicher said that the amount of synthetic DNA he detected in all three Australian vials “far exceeded” the allowable regulatory limit set by the Therapeutic Goods Administration (TGA).
Given scientific evidence suggesting that synthetic DNA can enter the cell nucleus and potentially integrate into the human genome, “it is important to investigate whether integration can take place in primary cells in the vaccinated population”, Dr. Speicher said.
The Australian study confirms independent lab findings of high levels of residual DNA in mod-RNA Covid vaccines from Germany, the U.S. and Canada, highlighting that this is a global concern.
The study
Residual synthetic DNA is a byproduct from the mod-RNA vaccine manufacturing process, and is allowed under TGA regulations in levels of up to 10 nanograms (ng) per vaccine dose, and in fragment sizes of up to 200 base pairs (bp).
The TGA denies that Covid mod-RNA vaccines are contaminated with synthetic DNA above 10ng per dose, but as high levels had been detected in vials from other regions, the legal team behind the GMO case commissioned this study to determine residual synthetic DNA levels in Australian vials.
PJ O’Brien & Associates arranged for shipment of three vials with chain of custody, one Moderna and two Pfizer, to Dr Speicher’s lab at the University of Guelph in Canada. The vials were shipped on dry ice and stored in the lab fridge on arrival. The Pfizer vials had tamper seals intact, while the Moderna vial had been half-used.
Dr. Speicher used two methods to test for residual DNA levels – flourometry and qPCR – each with their own advantages.
qPCR is the method preferred by regulators. It captures lower readings of DNA because it can fail to pick up small fragments of DNA below 200bp and it measures less than 1% of the residual DNA plasmid, with the other 99% being extrapolated mathematically. This means the reading has greater repeatability, but it gives a less complete picture.
A Moderna patent (2014) related to “removal of DNA fragments in [the] mRNA production process” acknowledges that the qPCR method of quantifying residual DNA only detects some target DNA molecules, but “does not measure all other smaller DNA molecules that are partially digested” by the enzyme used to break them down for the filtration process.
Using qPCR, Dr. Speicher detected synthetic DNA up to 15-fold above the TGA’s limit in both Pfizer lots, but the Moderna lot was compliant.
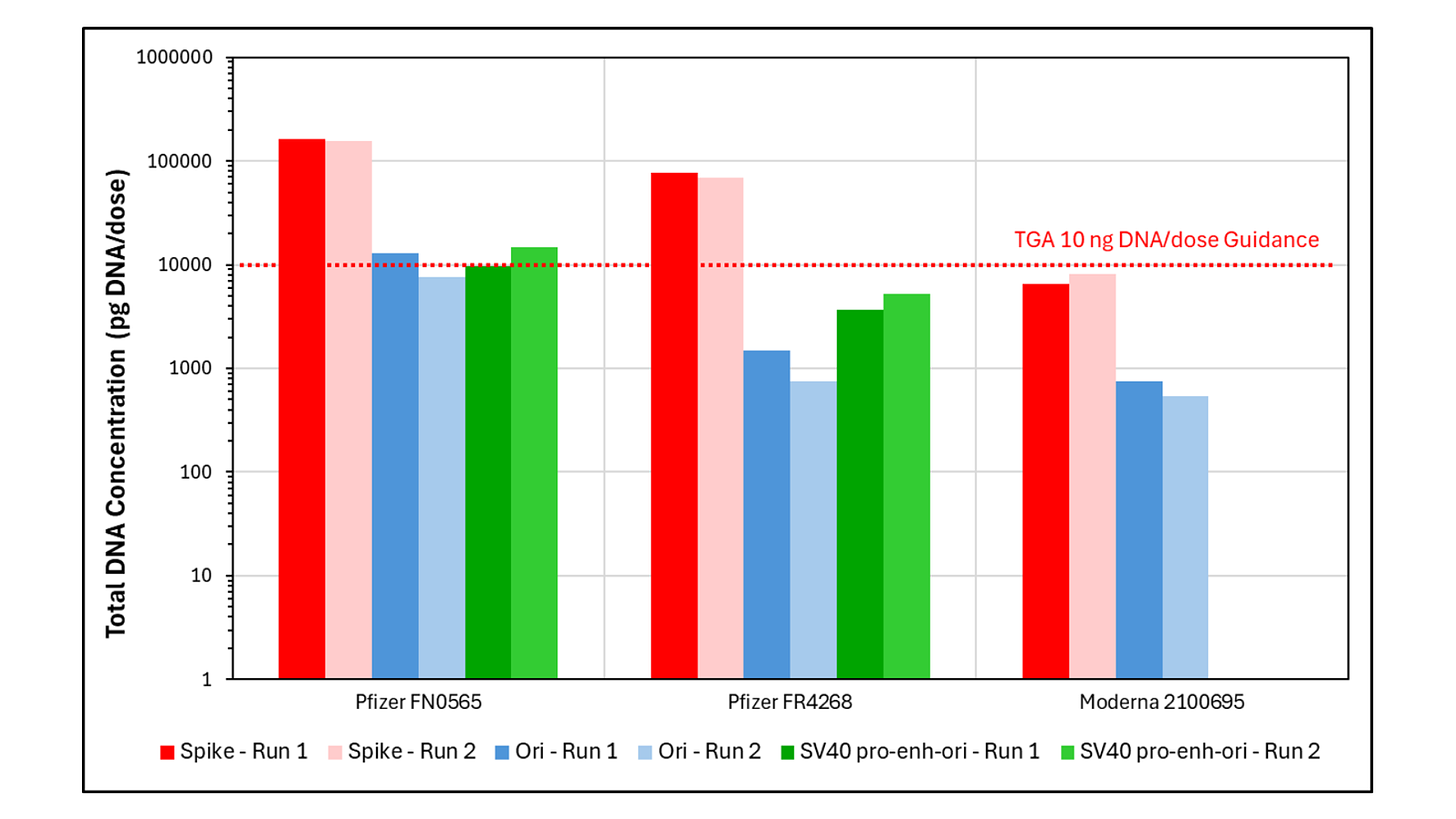
To prepare for flourometry testing, Dr. Speicher boiled the vaccines to dissolve the lipid nanoparticles (LNPs) encapsulating both the mod-RNA and residual synthetic DNA. This allowed for increased DNA yield in the reading.
However, there is the potential for “cross talk”, where mod-RNA can be accidentally included in the reading. To reduce cross talk, Dr. Speicher treated the samples with an enzyme called RNase A to degrade the mod-RNA, ensuring that it would not be picked up in the DNA reading.
Using flourometry, Dr. Speicher detected seven to 145-fold more synthetic DNA than the TGA’s 10ng limit. All vials exceeded the limit, with Moderna having the highest DNA load of 1460ng per dose.



