from The Epoch Times:
 Australian Comparative Data Reveals COVID-19 Vaccines Have the Country’s Largest Amount of Adverse Reactions
Australian Comparative Data Reveals COVID-19 Vaccines Have the Country’s Largest Amount of Adverse Reactions
The latest report on adverse reactions to vaccines in Western Australia has revealed that COVID-19 vaccinations have 24 times the rate of adverse reactions in the state compared to all other vaccines.
TRUTH LIVES on at https://sgtreport.tv/
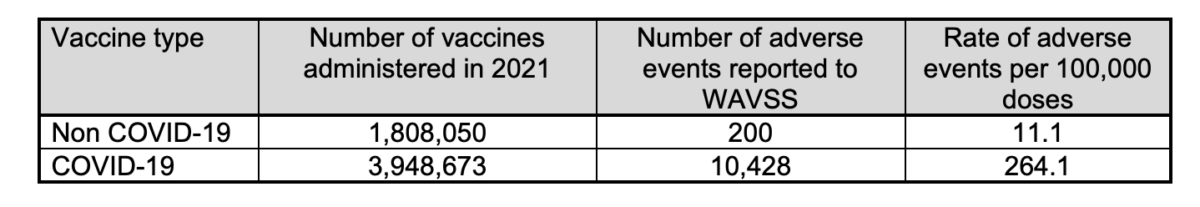
According to the state’s vaccine safety surveillance report (pdf), COVID-19 vaccines showed that for every 100,000 COVID-19 vaccines administered, 264 adverse events following immunisations (AEFIs) were recorded.
For all other vaccinations, 11.1 AEFIs were recorded, making the COVID-19 vaccines 23.8 times more likely than non-COVID-19 vaccines to result in adverse events.
The rate of adverse events varied among different types of COVID-19 vaccines.
The Spikevax (Moderna) vaccine recorded 281.4 AEFIs per 100,000 doses, Comirnaty (Pfizer) recorded 244.8, and the Vaxzevria (AstraZeneca) vaccine, which was removed from the vaccine program after reports emerged of blood clotting in younger people, recorded 306.
Adverse events following vaccination can range from mild, such as a sore arm, to serious conditions, such as anaphylaxis, thrombosis with thrombocytopaenia syndrome (TTS), Guillain-Barré syndrome (GBS), myocarditis, and pericarditis.
Collaboration Continues With 3-in-1 Super Jab
Meanwhile, despite these concerns, the Australian government’s partnership with Moderna to produce vaccines using experimental messenger RNA technology to prepare for the next pandemic means these vaccines are here to stay.
The company has been forming a trifecta jab to address the main respiratory viruses—influenza, COVID-19, and RSV to maintain its market share amid the falling revenue of vaccine companies as the health crisis subsides.
Moderna’s COVID-19 vaccine sales of US$18.4 billion in 2022 are expected to dive to $5 billion this year.
Recently, it was granted expedited approval by Australia’s authority for medicines for its mRNA-1345 (RSV vaccine), meaning that the company will be able to launch the vaccines in Australia before any other country in the world.
A spokesperson from Australia’s Therapeutic Goods Administration told the Epoch Times that Moderna was granted an accelerated approval process on March 30 after satisfying all of the following criteria:
- the medicine is new
- the medicine is for the treatment, prevention, or diagnosis of a life-threatening condition
- no other medicines that are intended to treat, prevent or diagnose the condition are included in the Australian drug register or there is substantial evidence that this medicine provides a significant improvement in efficacy or safety of the treatment, prevention or diagnosis of the condition compared to those goods already included in the register
- there is substantial evidence that the medicine provides a major therapeutic advance.



