from Sputnik News:

Neuralink’s ambitious goal is to create a seamless connection between human brains and computers, enabling individuals to communicate and interact with machines in unprecedented ways.
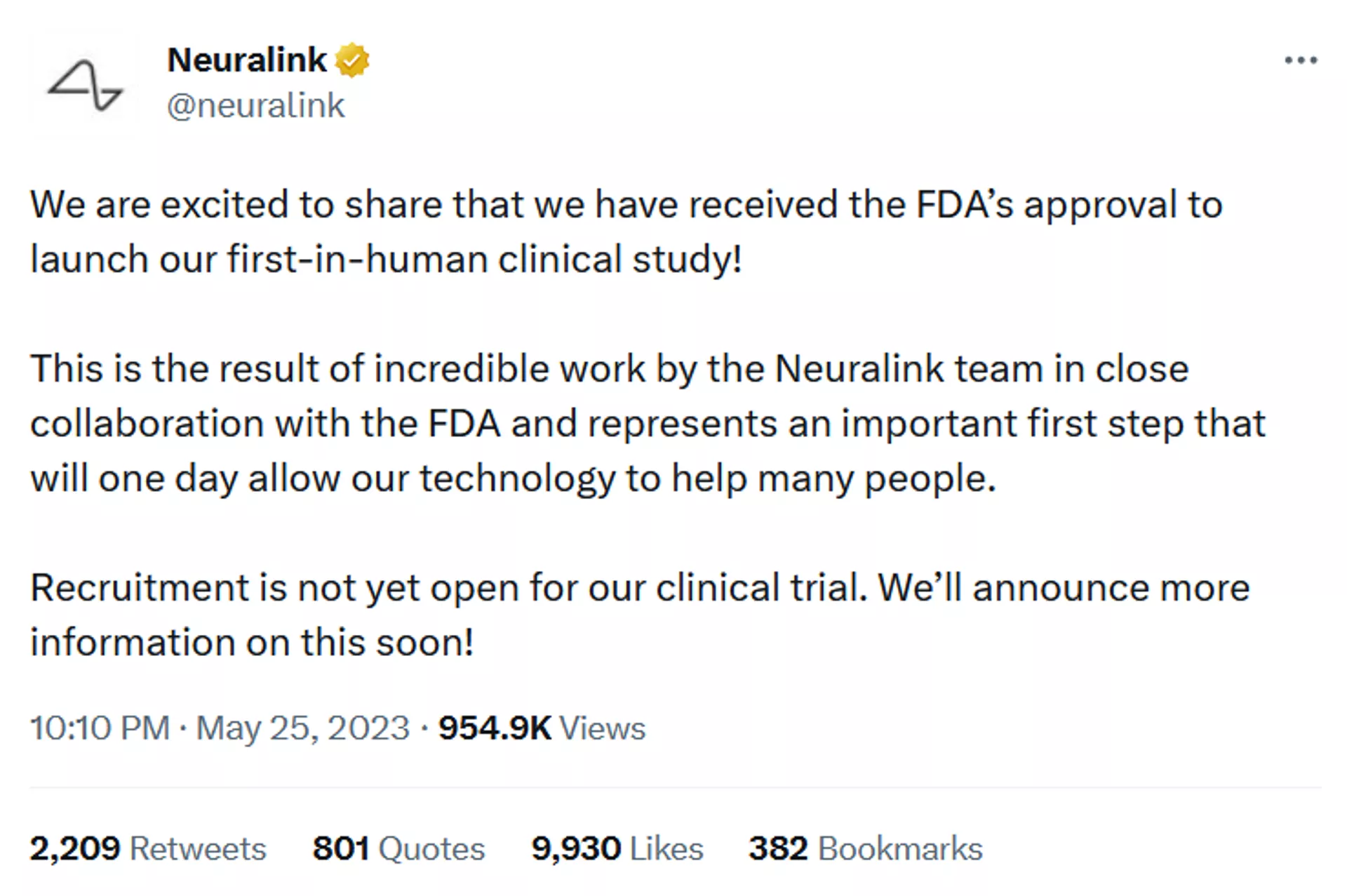
In a groundbreaking development for the field of neurotechnology, Neuralink, the brain-machine interface company founded by Elon Musk, has received the approval from the US Food and Drug Administration to conduct its first human trials.
TRUTH LIVES on at https://sgtreport.tv/
Neuralink is aiming to develop high-bandwidth interfaces that can be implanted into the human brain, allowing for bidirectional communication between the mind and external devices.
The FDA’s approval of Neuralink’s human trials signifies a significant vote of confidence in the company’s innovative technology and its potential to revolutionize the lives of individuals with neurological conditions and disabilities.
While the road to this milestone has been filled with challenges and rigorous testing, Neuralink has demonstrated promising results in preclinical studies, which have paved the way for advancing to human trials.
If successful, the cutting-edge technology could revolutionize various fields, including health care, communication, and human-computer interaction.
Potential applications range from helping individuals with neurodegenerative disorders, such as Parkinson’s or Alzheimer’s disease, to enhancing cognitive abilities and enabling novel methods of information processing.
The road ahead for Neuralink remains challenging, with many regulatory and ethical considerations to address. Ensuring the safety, privacy, and informed consent of participants will be of utmost importance throughout the trial process.