by Cullen Linebarger, The Gateway Pundit:

The Food and Drug Administration (FDA) has issued a dire warning to customers NOT to purchase more than two dozen eye drop products being sold at some of the nation’s most prominent retailers. While this is undoubtedly critical public information, this new revelation should also raise some serious questions about the FDA itself.
As ABC News reported Monday, the FDA in a press release Friday revealed that the eye drop products were manufactured in a facility with “insanitary conditions” and carry a “potential risk of eye infections that could result in partial vision loss or blindness.”
TRUTH LIVES on at https://sgtreport.tv/
These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses. FDA recommended the manufacturer of these products recall all lots on October 25, 2023, after agency investigators found insanitary conditions in the manufacturing facility and positive bacterial test results from environmental sampling of critical drug production areas in the facility.
The location of this facility is unknown at the present time. The FDA did not even specify if the plant’s location was in America
According to the FDA, the impacted products are marketed under the brands CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up&Up, and Velocity Pharma. The FDA shared a full list of the 26 products on its website.

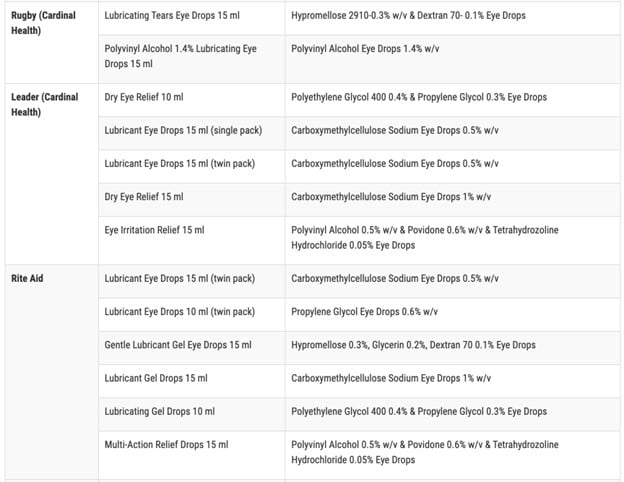
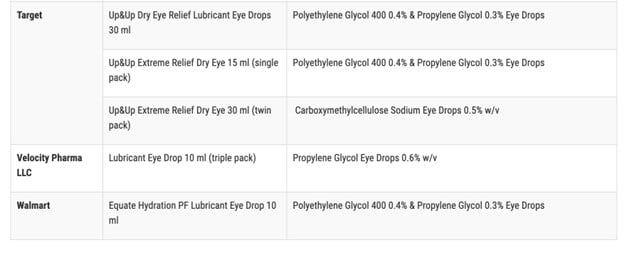
Anyone who purchased these products from these locations are recommended to “immediately stop using” them and dispose of them properly. This includes either dropping off the product at a drug take-back site or following the FDA’s steps to dispose of the product in the trash according to ABC News.
The FDA says they have yet to receive reports of eye infections caused by the products, but is urging health care professionals and patients to report any “adverse events or quality problems.”
Read More @ TheGatewayPundit.com



