by Amy Kelly, Daily Clout:
On September 5, 2023, DailyClout reported that a War Room/DailyClout Research Team – Corinne Michels, PhD; Daniel Perrier; Jeyanthi Kunadhasan, MD; Ed Clark, MSE; Joseph Gehrett, MD; Barbara Gehrett, MD; Kim Kwiatek, MD; Sarah Adams; Robert Chandler, MD; Leah Stagno; Tony Damian; Erika Delph; and Chris Flowers, MD – broke a huge story about there being more cardiovascular deaths in the vaccinated than in the unvaccinated in Pfizer’s clinical trial, as well as that Pfizer did not report the 3.7-fold cardiovascular adverse events signal and also delayed reporting deaths so that it favored the vaccinated arm of the trial. (https://dailyclout.io/report-84-warroom-dailyclout-research-team-breaks-huge-story/)
TRUTH LIVES on at https://sgtreport.tv/
Now, as a follow up to that report, DailyClout reveals that Jeyanthi Kunadhasan, MD, part of that same Research Team, found even more damning evidence showing that Pfizer delayed recording deaths in Case Report Forms (CRFs), which allowed the company to not report those deaths as part of its emergency use authorization (EUA) data filing with the Food and Drug Administration (FDA). Daniel Perrier from the Research Team and Dr. Kunadhasan found, analyzed, and verified the data supporting this discovery. It is very likely that had the deaths been recorded when Pfizer became aware of them and then accurately filed as part of the EUA dataset, the public would not have accepted an EUA had been granted by the FDA.
On December 10, 2020, one day before the FDA granted Pfizer’s EUA, Susan Wollersheim, M.D. of the FDA/Center for Biologics Evaluation and Research (CBER) Office of Vaccines Research and Review, Division of Vaccines and Related Products Applications, presented, “FDA Review of Efficacy and Safety of Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Request” at the CBER Vaccines and Related Biological Products Advisory Committee 162nd Meeting. On page 42 of the slide deck, Dr. Wollersheim presented:

The data showing two vaccine deaths and four placebo deaths as of December 10, 2020, was incorrect at the time of the EUA request presentation, and the correct data was available in Pfizer’s own documentation and, thus, also available to the FDA.
As of November 14, 2020, the data cutoff for the EUA dataset, Pfizer possessed data showing that the vaccine arm of the COVID-19 mRNA vaccine clinical trial had the same number of deaths as the placebo arm. In other words, there was no evidence of Pfizer’s COVID vaccine having a positive impact on death outcomes.
The Pfizer narrative descriptions of the deaths show that, though that the clinical trial sites had been informed by the deceased patients’ loved ones on the days of their deaths [October 19, 2020, and November 7, 2020 (pp. 71 and 75), well before the EUA data cutoff date in both cases], those two deaths from the vaccinated arm that were not included in the FDA presentation are listed below:


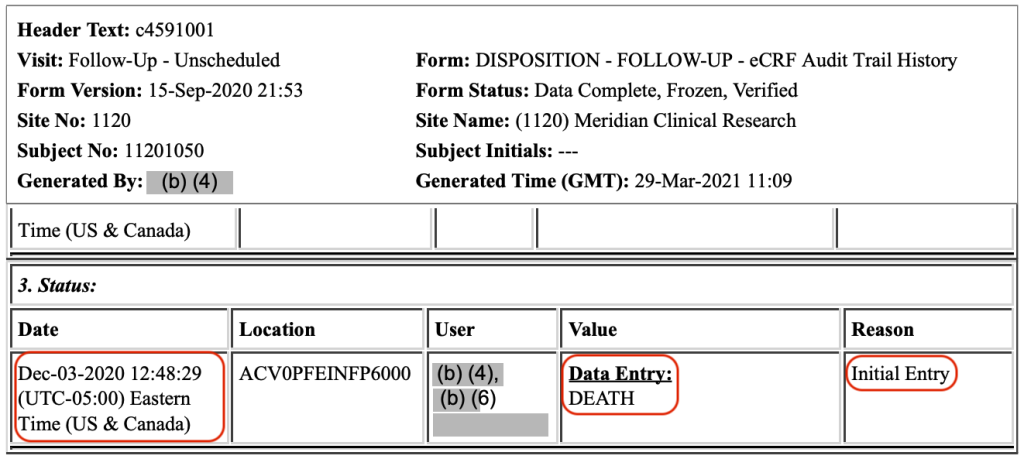
Case Report Forms (CRFs) capture patient data during a clinical trial. The CRFs for these two patients, Subjects 11141050 and 11201050, do not mention that Pfizer knew of their deaths on their dates of death. Rather, Subject 1141050’s death was first entered into the CRF on November 25, 2020, at 18:51:46 Central Time, 37 days after the patient’s death.

And, Subject 11201050’s death was first entered into the CRF on December 3, 2020, at 12:48:29 Eastern Time, 26 days after the patient died:

It appears that Pfizer not entering the death dates of the above two Subjects into their CRFs when Pfizer first learned of them – i.e., on the dates of death – allowed the company to hide the deaths until the EUA data cutoff date had passed. To summarize what was known and important dates:

According to Pfizer’s own protocol, reporting of deaths should have occurred within 24 hours:
- Section 8.3.1.1. “Reporting SAEs to Pfizer Safety” states, “All SAEs occurring in a participant during the active collection period as described in Section 8.3.1 are reported to Pfizer Safety on the Vaccine SAE Report Form immediately upon awareness and under no circumstance should this exceed 24 hours, as indicated in Appendix 3 [pp. 1271-1277]. The investigator will submit any updated SAE data to the sponsor within 24 hours of it being available.” [Emphasis added.] [p. 74]
- Additionally, Section 8.3.1.2. “All nonserious AEs and SAEs occurring in a participant during the active collection period, which begins after obtaining informed consent as described in Section 8.3.1, will be recorded on the AE section of the CRF. The investigator is to record on the CRF all directly observed and all spontaneously reported AEs and SAEs reported by the participant.” [Emphasis added.] [p. 74]



