by Heather Hudson, Daily Clout:
The below work was written from March to May 2023; after working with attorneys and physicians to bring this information to the attention of our lawmakers, the below introduction and eight-page Executive Brief/synopsis accompany a 90-page executive summary that was sent to lawmakers, and more through the summer. These writings include references, quotes, and citations (last page) to the manufacturer’s and LNP developers’ published writings, which show their own concerns with the LNPs published as late as July 2020, just months before the EUA approval of the Covid-19 mRNA LNP-based vaccines. It also provides studies from the LNP development (and LNP developers) and extensive studies and documentation in medical journals on the pre-pandemic known reactions to the lipids in drugs that Pfizer lists as having similar lipid nanoparticles in its own internal documents and more. This series will be presented in several sections like the Twitter Files; these are the LNP Files.
TRUTH LIVES on at https://sgtreport.tv/
###
Synopsis and Executive Brief to “Executive Summary on Sars-CoV-2 mRNA Vaccine (injectable therapies) Lipid Nanoparticles (LNPs) and Known Pre-pandemic Lipid and Liposomal CARPA (Complement Activated Reaction PSEUDO ALLERGY) and mRNA Covid-19 Vaccine Adverse Events, Myocarditis, and Autoimmunity”
May 2023 – Heather Hudson
Introduction:
1. In 2020, the Lipid Nanoparticle was a little-known and unknown science according to the Pfizer Covid-19 mRNA vaccine LNP co-developer: When the Covid-19 mRNA vaccines were approved under the Emergency Use Authorization (EUA), the consumer could not have known about these highly specific areas of nanomedicine and nanotechnology. The LNPs, studies, and development had been a little-known or niche area of science and medicine that was struggling to find its footing, funding, and success in treatment (largely due to safety issues (as seen in peer-reviewed articles within the attached Executive Summary)). Nano lipids were mainly used as carrier lipids in cancer drugs. In some of the drugs, patients were pre-medicated to avoid the lipid adverse reactions. LNPs had little recognition, as seen here, in this Cell Magazine article in which Acuitas labs co-founder and Covid-19 mRNA-LNP vaccine LNP co-developer Peter Cullis is interviewed, in 2022, and states, “Then in November [2020] … lipid nanoparticles became really popular. Nobody had ever heard of a lipid nanoparticle before, but in the last year and a half, they certainly have.”[11] More information can be found on the illusive nature of the use of pre-pandemic nanomedicine in references [12] and [23], and more can be found in quoted material within the attached Executive Summary.
2. What is an LNP? The lipid nanoparticle (LNP) can be thought of like a bubble that surrounds the drug or therapy (payload) that is to be delivered in the cells (for example mRNA gene therapy). The LNP bubble shape forms as numerous cone shaped individual lipid molecules attract to one another to form a bubble shape with a hollow center, which then houses the payload which can also include more LNPs along with gene therapy such as mRNA, siRNA, etc.
3. What do LNPs do? The LNPs provide a means for the therapy or drug to be carried inside the LNP bubble to travel in our body and enter our cells that are naturally surrounded by their own lipid layer. The cells allow the LNP in via an exchange of charges of the components when the LNP lipid and the natural cell lipid join. Inside the cell, the LNP falls apart and releases its payload. As seen in the medical studies sometimes the LNPs do not enter the cell (as they were intended to), or they are not adsorbed (due to adsorption rates and possibly the biomolecular or protein corona as seen below) and they can circulate in the body instead of entering the cell. Also, per the medical studies, sometimes the LNPs can fall apart before being taken up by the cells and the lipids circulate in the body. For the LNPs that enter cells, according to this study, “The apparent terminal t½ in plasma likely represents the re-distribution of the respective lipids from the tissues into which they have distributed as the LNP back to plasma where they are eliminated.”[44][75]
4. What is PEG? The LNP capsules often contain PEG (Polyethylene Glycol) which is an emulsifier that helps to keep the lipids stable and is meant to prolong the life of the LNPs and to stabilize the LNP bubble structure. Some people have a true allergy to PEG.
5. PEG and LNP safety issues: PEG is found in the medical literature to be immunogenic, and aside from PEG, the shape, size and biophysical properties (biomolecular corona, etc.) of the LNPs can elicit unwanted immune response which can be seen through the liposomal and LNP development history as the body mistakes nano sized molecules of a certain size for virus molecules. [72]
6. Unpredictable LNP adverse events: Beyond PEG allergy, PEGylated LNP molecules have a separate history of adverse reactions that are unpredictable, such as Complement Activated Pseudo Allergies or CARPA, autoimmune activation, coagulation dysregulation and more which are detailed and referenced in the Executive Summary.
7. Pre-pandemic lipid adverse reactions – LNP and lipid adverse reactions are NOT just an allergy; they can be deadly and can elicit complement-activated immune dysregulation (autoimmune disorders, and more): Certain past liposomal and LNP drugs have required pre-treatment with steroids, antihistamines and more to prevent adverse events brought on by the complement system such as pseudo allergies, CARPA, pseudo-HSRs and IRs or infusion reactions. Some of these lipids are described as “similar lipids” to the Covid-19 mRNA vaccines LNPs by Pfizer. The patients who take the LNP carrier drug with “similar lipids” (to the Pfizer Covid-19 vaccines lipids) were pre-treated for these reactions in the clinical trials, and these patients for this drug are pre-treated upon administration of this drug to date. These specific reactions occur at a “very common” and “common” rate per the FDA fact sheet for this drug and the ADR, and they were known pre-pandemic to Pfizer/BioNTech mRNA and LNP co-developers. They were not warned of or listed by name in the FDA EUA patient and provider fact sheet.
8. Unrecognized high rate of reaction: In 2018, Janos Szebeni, who learned to make liposomes in a lab with (the now senior VP of BioNTech Katalin Kariko), conducts LNP animal studies and more. He co-authored the following article on the role of complement activation in HSRs [Hypersensitivity Reactions, AKA IRs, IRRS, Infusion reactions and CARPA (pseudo allergy)], which explains, “HSRs have been traditionally categorized in four groups, … However, it has increasingly been recognized that a substantial portion of acute allergic reactions, whose symptoms fit in Coombs and Gell’s Type I category, are actually not initiated or mediated by pre-existing IgE antibodies. These reactions are known to be “pseudoallergic” or “anaphylactoid.” There are estimates that pseudoallergy may represent as high as 77% of all immune-mediated immediate HSRs…, implying hundreds of thousands of reactions and numerous fatalities every year… Many of these reactions involve the activation of the complement system, an essential humoral arm of innate immunity. Complement activation-related pseudoallergy (CARPA) is linked to adverse events evoked by several liposomal and micellar formulations, nanoparticles, radiocontrast agents, and therapeutic antibodies…”[81]
9. Relatively unknown reactions outside of the nanomedicine field: In this 2020 Science magazine article, written just after the Covid-19 mRNA vaccines saw at least eight people develop severe allergy-like reactions, it states, “Szebeni says the mechanism behind PEG-conjugated anaphylaxis is relatively unknown because it does not involve immunoglobulin E (IgE), the antibody type that causes classical allergic reactions. (That’s why he prefers to call them “anaphylactoid” reactions.) Instead, PEG triggers two other classes of antibodies, immunoglobulin M (IgM) and immunoglobulin G (IgG), involved in a branch of the body’s innate immunity called the complement system…”[6]
10. Pre-pandemic reactions resemble current reactions: This 2022 study states, “… the present study focused on the HSRs to Comirnaty, as it consists of PEGylated LNPs that resemble PEGylated liposomes, and “lessons” learned from nanomedicine research suggest that such nanoparticles (NPs) injected into the blood can cause so-called “infusion reactions” whose symptoms are very similar, or the same as those reported for the HSRs to LNP-mRNA vaccines …”[28]
11. Unpredictability and the Need for Premedication: This 2018 article, “Roadmap and strategy for overcoming infusion reactions to nanomedicines” by Szebeni et al. states, “Despite decades of research suggesting that the incidence of IRs depends on both pharmacodynamics and pharmacogenetics, it is largely unknown why some patients develop these reactions while others do not. The lack of uniform terminology and classification of the reactions further complicates the issue. As such, acute IRs cause substantial stress among patients and their families as well as care providers and regulatory agencies… Therefore, IRs in patients are currently managed by systemic administration of immunosuppressive, anti-pyretic, and anti-inflammatory medications before the infusion, during administration, or both…”[9] Again, these dysregulated immune reactions and adverse events have now been seen in the Covid-19 mRNA-LNP vaccines, including acknowledgement by the Mayo clinic and other physicians in case studies and more. [2][53-57][91] Reference [57] includes thirty-one cases of new-onset autoimmune disorders after Covid-19 vaccines; twenty-nine of these cases are attributed to Pfizer, and one of these cases was reported as having a fatal outcome, per the study.
12. PEG and LNP antibodies: This 2022 study, states, “The first injection of PEGylated drugs induces anti-PEG antibodies, which then bind and form an immune complex with the second dose of the PEGylated compound to activate the complement system. This results in the opsonization of PEG with C3 fragments and enhanced uptake by Kupffer cells in the liver and can result in altered drug pharmacokinetics and biodistribution and reduced drug efficacy in subsequent doses.” [86]
13. Circulation time pharmacokinetics, kinetics, and biodistribution in relation to adverse events: The LNP circulation time within the body and the biodistribution of the LNPs can play a part in these adverse reactions. Biodistribution studies and animal studies are crucial to determining the safety of the LNPs, as is seen in peer-reviewed studies within the Executive Summary.
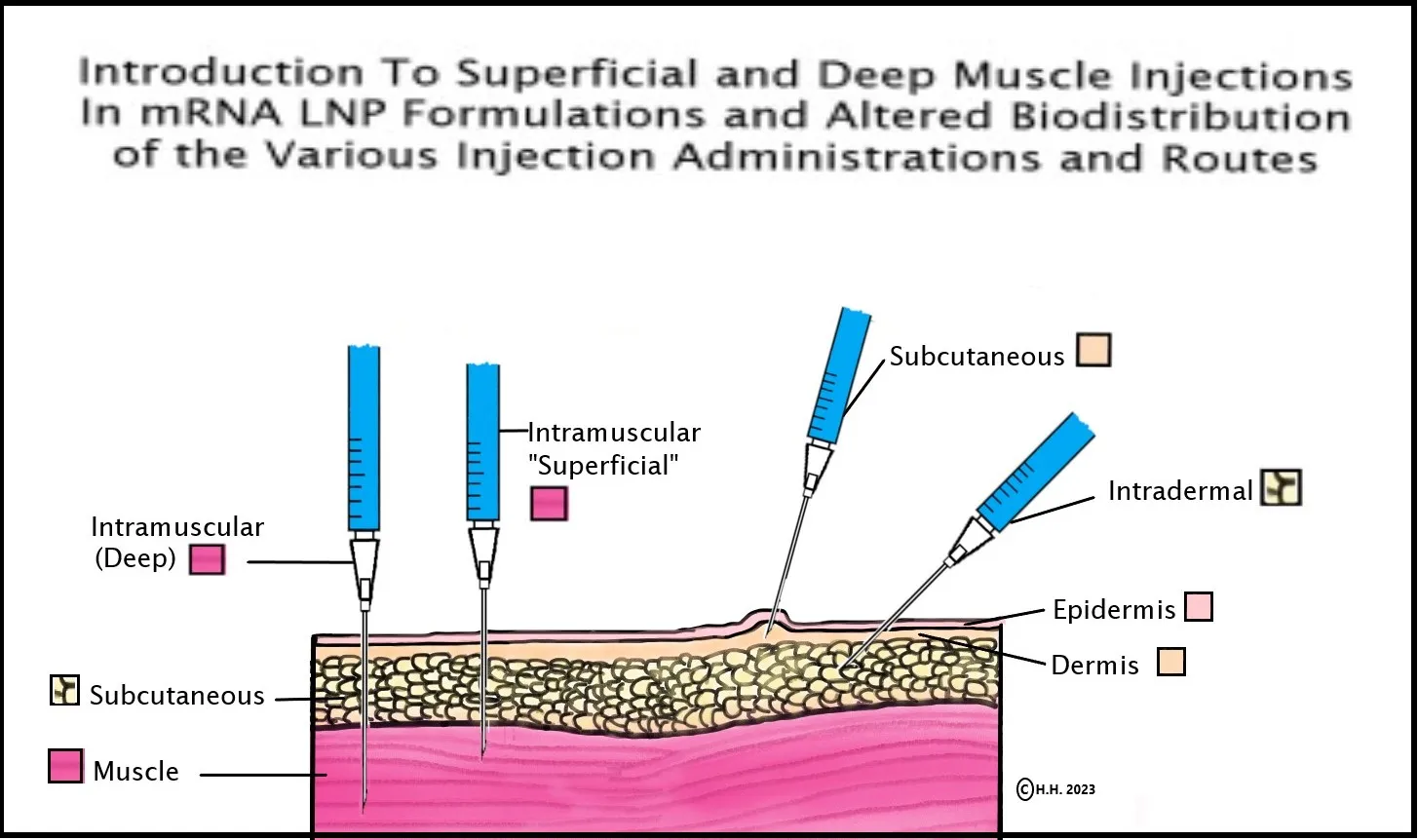
14. Pfizer/BioNTech & Acuitas Therapeutics scientists show in their 2015 study that mRNA-LNP intramuscular injection depth has the ability to alter biodistribution and determine whether injections disseminate to the liver or stay in the arm/muscle: In 2015, a BioNTech VP and a co-mRNA developer, along with four scientists from the Acuitas Therapies lab (the lab that created the Pfizer Covid-19 vaccine LNPs) discovered that biodistribution of the mRNA-LNP formulation could be altered via the route of delivery of the mRNA-LNP in the body, but what is especially relevant is the depth of the muscle could determine whether the formulation distributed mainly to the liver or remained in the muscle tissue, as seen here in the study where it states, “Here, we show that in vivo administration of mRNA-LNP complexes by various commonly used injection routes displays different expression patterns and kinetics. Depending on the site of injection, both local protein production and dissemination to the liver occurs. Interestingly, we observed that with intramuscular injection, the depth into the muscle also determined whether the majority of protein produced was in the muscle, for superficial injections, or in the liver, for deep muscular administration (unpublished observations). Importantly, very low doses (0.005 mg/kg) of mRNA-LNPs could be translated for several days following the tested delivery routes… “[58] This information on the distribution of mRNA and LNPs in the body was not provided by Pfizer in their FDA EUA information to patients and providers.
See diagram and credit/link to this page if re-posting.
 100vw, 1024px” srcset=”https://dailyclout.io/wp-content/uploads/injections-mrna.jpeg 1024w, https://dailyclout.io/wp-content/uploads/injections-mrna-300×178.jpeg 300w, https://dailyclout.io/wp-content/uploads/injections-mrna-768×455.jpeg 768w, https://dailyclout.io/wp-content/uploads/injections-mrna-600×355.jpeg 600w” alt=”” width=”1024″ height=”607″ data-src=”data:image/svg+xml,%3Csvg%20xmlns=’http://www.w3.org/2000/svg’%20viewBox=’0%200%201024%20607’%3E%3C/svg%3E” data-srcset=”https://dailyclout.io/wp-content/uploads/injections-mrna.jpeg 1024w, https://dailyclout.io/wp-content/uploads/injections-mrna-300×178.jpeg 300w, https://dailyclout.io/wp-content/uploads/injections-mrna-768×455.jpeg 768w, https://dailyclout.io/wp-content/uploads/injections-mrna-600×355.jpeg 600w” data-sizes=”(max-width: 1024px) 100vw, 1024px” data-was-processed=”true” /></picture>
<div class=)




