by Robert W. Chandler, MD, MBA, Daily Clout:
Summary
Pfizer Report 70 introduced CoVax Disease, a new class of illness that has arisen from the use of mRNA COVID vaccines. CoVax Disease encompasses multiple pathological mechanisms, presents with multi-organ/system involvement, and affects all age groups. This article will focus on the very negative medical impacts on children’s health after receiving mRNA vaccines. Additionally, sex-related effects will be presented.
COVID-19 was a disease primarily of older people and those with comorbidities. Unfortunately, the experimental gene therapy products known as COVID “vaccines” have devastating effects in young, healthy people of both sexes and all ages from conception upward through the age brackets.
TRUTH LIVES on at https://sgtreport.tv/
Young men have more myo/pericardial disease and more fatalities. Females have more disability and more adverse events overall. As the Cho et al. and Barmada et al. studies document, long-term cardiac disease is one outcome from these drugs.
Disease categories associated with gene therapy products are diverse, are unusual in many cases, and can be unusually severe as in MIS-C, sudden death/cardiac arrest, stroke, and turbo cancer, as illustrated by the intracardiac epithelioid sarcoma case reviewed earlier in this report. Children are not exempt from these illnesses.
Causation is a complicated topic. However, there is ample support presented herein for concluding that some or many of the medical conditions that arise following an injection of C19 gene therapy products were caused by them.
I. Introduction
Since the early medical papers emerging from China in January 2020, it was apparent that the SARS-CoV-2 (SC2) virus preferentially produced disease, COVID-19 (C19), in the elderly with comorbidities. (doi:10.1001/jama.2020.1585) Some younger people with comorbidities also were at risk but the risk for severe disease and death in the general population was low. (https://doi.org/10.1016/S0140-6736(20)30211-7)
Lipid-nanoparticle coated mRNA drugs (LNP/mRNA) were developed to prevent C19 disease. These drugs along with the adenovirus-vectored products will be referred to collectively as gene therapy products (GTPs). Pfizer Confidential 5.3.6 (https://www.phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf) documented COVID-19 as an Adverse Event following inoculation with Pfizer’s BNT162b2 gene therapy product as well as over 140,000 additional adverse events reported in the first ten to twelve weeks. (https://dailyclout.io/pfizer-evidence-so-far-coverups-heart-damage-and-more/)
The object of this paper is to review Adverse Event reports following LNP/mRNA gene therapy treatments pertinent to differential age and sex reporting with specific coverage of the zero- to 18-year-old demographic.
Specific clinical features of medical conditions afflicting America’s youth following injection of Spike-mediated genetic treatment will be discussed. The article will conclude by reviewing the time course of Vaccine Adverse Event Reporting System (VAERS) event reporting relative to United States (U.S.) dosing data. VAERS reports are for the U.S. and its Territories unless otherwise specified.
The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) maintain VAERS, which is a public resource. (https://wonder.cdc.gov/controller/datarequest/D8) Open VAERS harvests data from VAERS in specific categories and is useful for summary data. (https://openvaers.com/covid-data)
In addition to VAERS and Open VAERS, this article will make use of case and small series reports from the medical literature, the FDA-released Pfizer Confidential Documents archive (https://phmpt.org/pfizer-16-plus-documents/), and reporting from individuals including William Makis, M.D., Professor Mark Crispin Miller, Ed Dowd and his partners, Jessica Rose, and others.
As a caution, the limitations of VAERS are spelled out on the CDC website:

Reports are not detailed enough for many determinations (1), but the utility of the system is as an early warning system (2). Much of the VAERS system activity is invisible to the user, such as what activity occurs from the time a report is filed with the CDC to when it gets posted and after its initial posting. The numbers that are given in response to queries change over time as the system is updated at least every week. Follow-up data are obtained but not posted.
Events vary in detail from diagnosis only to detailed reporting of medical data. Some categories return cryptic data like “No Adverse Event,” of which there were 32,072 reports for C19 products from 2020 through mid-June 2023 for all ages with 1,003 associated events including 13 deaths.
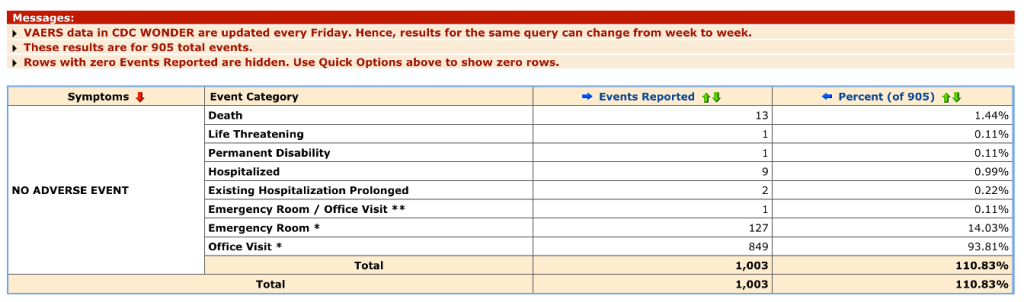
Here is the information of one fatal case so designated. VAERS ID 1205421 was a 61-year-old male who died less than two days after receiving his second dose of mRNA1273 (Moderna). Someone made the effort to file the report but with no details about this man’s death.

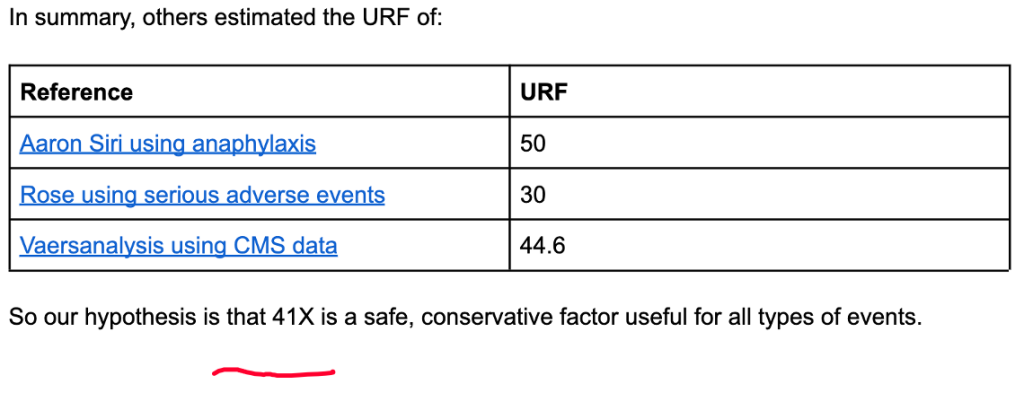
VAERS numbers are generally considered to represent only a fraction of the actual prevalence of the medical conditions reported and for this reason attempts have been made to estimate the true number by estimating what is called the Under Reporting Factor (URF). True prevalence data are hard to find.
Steve Kirsch, Jessica Rose, and Mathew Crawford performed detailed calculations of URF concluding that an URF of 41 was justified. (https://www.skirsch.com/covid/Deaths.pdf, https://jessicar.substack.com/p/the-under-reporting-factor-in-vaers)
II. C19 Gene Therapy: Age and Sex Effects
A. BNT162b2 and mRNA1273 (LNP/mRNA) Approval Schedule
For reference, the following dates are noted:
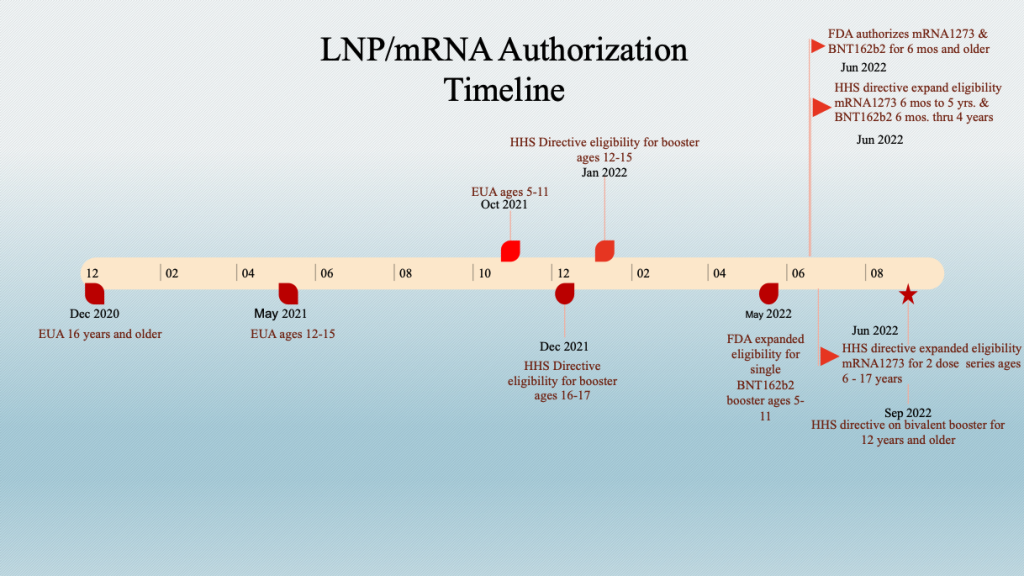
The staggered inoculation of age groups should be kept in mind when looking at data sets that combine age groups. This stagger adds an element of complexity when time series analyses are conducted across age groups.
Example: The age brackets in VAERS do not match the age brackets used for the “vaccine” dosing schedule. However, the six-month-old to 5-year-old group has bracketing close to the corresponding dosing brackets.
Five-year-olds became eligible to receive BNT162b2 on October 29, 2021, and both mRNA drugs were released for six-month-olds to four-year-olds for BNT162b2 and five-year-olds for mRNA1273 on June 18, 2022. The histogram below is a plot of adverse events according to the inoculation schedule showing a spike in event reports following the two authorization dates.



