by Michael Nevradakis, Ph.D., Childrens Health Defense:
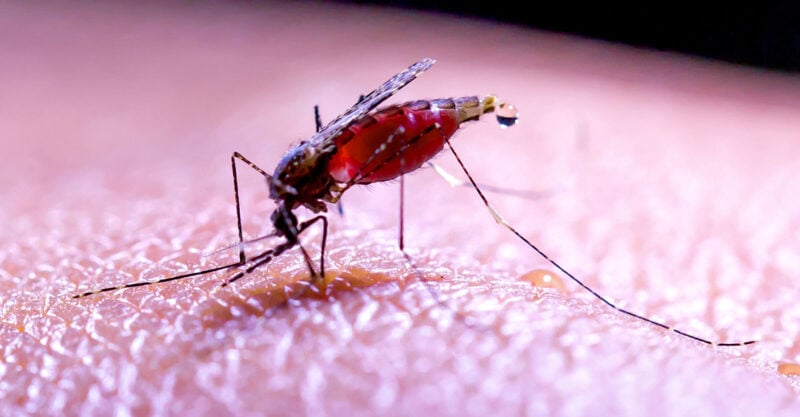 The National Institutes of Health funded a malaria vaccine trial study that used genetically modified mosquitoes to “vaccinate” humans. The Bill & Melinda Gates Foundation has close ties to the research.
The National Institutes of Health funded a malaria vaccine trial study that used genetically modified mosquitoes to “vaccinate” humans. The Bill & Melinda Gates Foundation has close ties to the research.
The National Institutes of Health (NIH) funded a malaria vaccine trial study that used genetically modified (GM) mosquitoes to “vaccinate” humans.
TRUTH LIVES on at https://sgtreport.tv/
A team of researchers at the University of Washington conducted the study, which was published in the Science Translational Medicine journal.
The study involved 26 participants who received three to five “jabs” — or bites from a small box containing 200 GM mosquitoes — over a 30-day period.
Sanaria, a company funded in part by the Bill & Melinda Gates Foundation (BMGF), is closely connected to the research, and the researchers involved in the trial use a gene-editing technology heavily promoted by Bill Gates.
Genetically modified mosquitoes used as ‘flying syringes’
The trial used malaria-causing Plasmodium mosquitoes that were genetically modified to avoid causing sickness in humans to infect participants with a “minor” version of malaria — insufficient to cause severe illness, but enough to make the humans create antibodies.
Dr. Sean Murphy, lead author of the study, told NPR, “We use the mosquitoes like they’re 1,000 small flying syringes.”
Despite the publicity generated by this study, however, results appear to have been mixed.
Of the 14 trial participants exposed to malaria, seven contracted the disease. For the remaining seven, the protection conferred by the “vaccine” did not last more than a few months and eventually dissipated.
According to the study:
“Half of the individuals in each vaccine group did not develop detectable P. falciparum infection, and a subset of these individuals was subjected to a second [Controlled Human Malaria Infection] 6 months later and remained partially protected.”
According to the Centers for Disease Control and Prevention (CDC), “infections caused by P. falciparum are the most likely to progress to severe, potentially fatal forms” of malaria.
Adverse reactions in trial participants reportedly were “what one would expect after getting bit by hundreds of mosquitoes and nothing more.”
For example, trial participant Carolina Reid told NPR her entire forearm “swelled and blistered.”
Despite the study’s mixed results, the researchers claimed the “results support further development of genetically attenuated sporozoites as potential malaria vaccines.”
The researchers suggested several reasons for using live mosquitoes rather than a vaccine that could be delivered via a syringe, including that the use of live insects made sense, as the P. falciparum parasite quickly matures inside the mosquito.
In addition, the process of developing a version of the parasite that could be delivered via a syringe was described as “costly and time consuming.”
Nevertheless, according to Murphy the study will not be used for the mass vaccination of humans. However, the researchers involved in the trial said they believe the approach they used can eventually result in the development of a “substantially more effective” malaria vaccine.
At present, only one malaria vaccine is in use. The RTS,S vaccine produced by GlaxoSmithKline was approved by the World Health Organization in October 2021, but reportedly has an efficacy rate of only 30-40%.
Dr. Kirsten Lyke, a vaccine researcher at the University of Maryland, described the use of a genetically modified live parasite as a vaccine as “a total game changer,” saying the team of researchers “went old school with this one.”
“All things old become new again,” Lyke told NPR.
Lyke, who was not involved in the GM mosquito malaria trial, led the Phase 1 trials for the Pfizer/BioNTech COVID-19 vaccine and also served as co-investigator for COVID-19 vaccine trials administered by Moderna and Novavax.
Stefan Kappe, a parasitologist at the University of Washington and the Seattle Children’s Research Institute — who was one of the authors of the study — said that the approach described by Lyke is already being worked on by the team, adding that the team believes “we can obviously do better.”
Read More @ ChildrensHealthDefense.org



